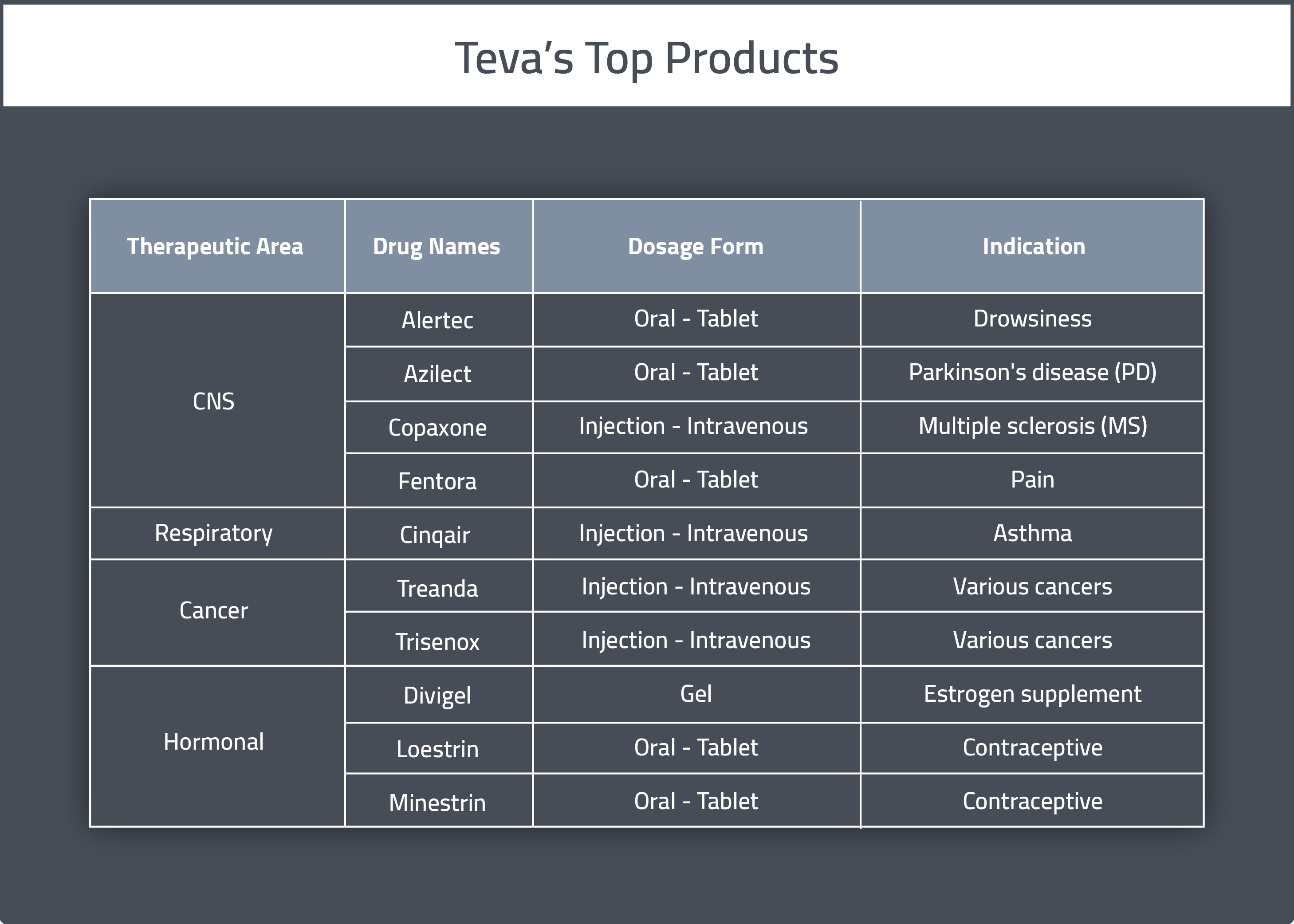
Huntington's disease drug: Teva Pharma settles patent dispute with Lupin, continues to litigate against Aurobindo
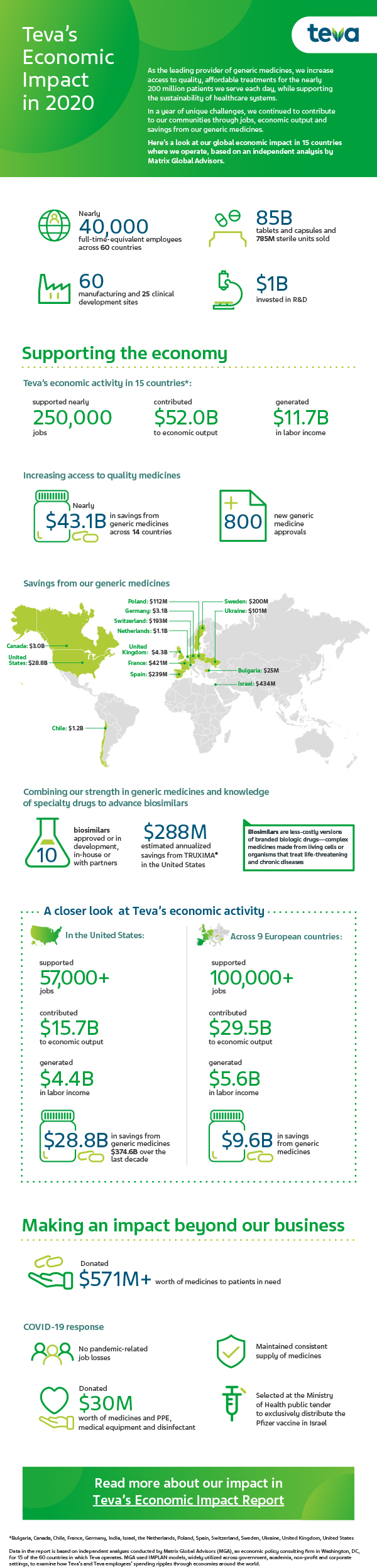
Teva Generic Medicines Saved the United States $28.8 Billion in 2020, and a Total of $43.1 Billion Across Major Markets, According to Independent Analysis | Business Wire

Lupin gets US health regulator's approval for asthmatic symptoms prevention drug - The Economic Times

Glenmark Pharma receives USFDA approval for manufacturing blood pressure tablets - Dalal Street Investment Journal

PDR0000401 An electronic inhaler add-on module which store and transmit information related to inhaler use (eModule) Attestation Statements Letter of Agency Teva Branded Pharmaceutical Products R&D;, .

PDR0000401 An electronic inhaler add-on module which store and transmit information related to inhaler use (eModule) Cover Letter Letter of Short Term Confidentiality Teva Branded Pharmaceutical Products R&D;, .
PDR0000401 An electronic inhaler add-on module which store and transmit information related to inhaler use (eModule) Cover Letter FCC_AntiDrug-Confidentiality_Signed Teva Branded Pharmaceutical Products R&D;, .